Amyloid Fibrils
Amyloid fibrils are peptide or protein aggregates that form under certain conditions in vitro or in vivo. For example, the amyloid fibril plaques found in brain tissue of Alzheimer patients are formed from the peptide Aβ and are associated with neurodegeneration. Amyloid formation is also observed with other diseases, such as type II diabetes and Creutzfeldt-Jakob disease.
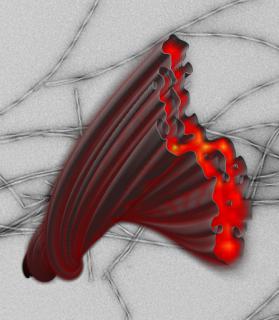
Amyloid structures represent an alternative to the native folding pattern of many peptides and proteins. A characteristic motif of this folding pattern is the cross-β structure in which the peptides or proteins associate by β-sheet formation within protofilaments making up a fibril.
In collaboration with Marcus Fändrich (Ulm University, Germany), we study the molecular architecture of amyloid fibrils associated with human disease. Our goal is to identify fundamental principles of amyloid formation, and potential targets for disease treatment. The Figure shows an 8 Å structure of an Aβ(1-40) fibril [1]. At this resolution, the path of the peptide can be identified.
Other publications on the topic: [2][3][4][5][6][7][8][9][10][11][12][13]
References
2008Proc Natl Acad Sci U S A1057462-6
2006J Mol Biol362347-54
2007J Mol Biol371812-35
2008Proc Natl Acad Sci U S A1057462-6
2009J Mol Biol386869-77
2009Proc Natl Acad Sci U S A1064719813-8
2010Angew Chem Int Ed Engl491321-1323
2011Trends Biochem. Sci36338-45
2015Proc Natl Acad Sci U S A11211858-11863
2016Proc Natl Acad Sci U S A1136200–6205